August 26, 2025
Tag:
1 Preface
Capsicum red is a tetriterpenoid orange-red pigment found in mature red pepper fruits, belonging to the carotenoid class of pigments. Capsicum red pigment not only has a bright color and high color value, but is also widely used in the coloring of various foods such as aquatic products, meats, pastries, salads, canned foods, and beverages. Moreover, it can effectively extend the shelf life of simulated foods. Moreover, it is highly safe, has nutritional and health care effects, and has been proven by modern science to have functions such as anti-cancer and anti-radiation. It has a very promising future. Acid value, also known as neutralization value, acid value or acidity, is a measurement standard for the number of free carboxylic acid groups in a compound or mixture. Under the conditions of fat production, acid value can be used as an indicator of the degree of hydrolysis. The smaller the acid value, the better the quality and refining degree. Since the color of capsicum red itself can affect the color judgment of the indicator, this paper uses potentiometric titration to detect the acid value of capsicum red.
2. Instruments and reagents
2.1 Instruments
JH-T6 fully automatic potentiometric titrator, pH non-aqueous composite electrode, analytical balance
2.2 Reagents
Potassium hydroxide solution (0.1mol/L), isopropyl alcohol: ether =1:1
3 Experimental Methods
3.1 Sample Testing
Weigh about 3g of the sample into a titration cup, add 50mL of the ether - isopropanol (1+ 1) mixed solvent, mix well, and then use 0.
Titrate 1mol/L potassium hydroxide solution to the endpoint.
3.2 Parameter Setting
|
Titration mode : |
Dynamic titration |
Potential spike : |
300 |
|
Electrode equilibrium time |
8s |
Pre-controlled pH value : |
9.8 |
|
Electrode equilibrium potential: |
0.5mv |
Titration speed: |
Standard |
|
Minimum addition volume : |
0.01mL |
Pre-titration addition volume : |
0mL |
|
Final volume : |
20mL |
Stirring time after pre-titration : |
1s |
4. Results and Discussion
4.1 Experimental Results
Blank volume: 0.02mL, potassium hydroxide concentration: 0.1001mol/L
|
Sample name |
sampling quantity ( g ) |
titration volume
( mL ) |
acid value
( mg/g ) |
flat all values
( mg/g ) |
|
Pepper Red 1 |
3.1865 |
5.647 |
9.91 |
9.91 |
|
3.32255 |
5.894 |
9.92 |
||
|
3.1946 |
5.664 |
9.91 |
||
| Pepper Red 2 |
3.2810 |
10.882 |
18.57 |
18.59 |
|
2.5007 |
8.322 |
18.62 |
Calculation formula
Jiahang
In the formula:
X - Acid value content, unit: milligrams per gram (mg/g);
V - The volume of potassium hydroxide standard solution consumed by the sample, in milliliters (mL);
V0 - Volume of potassium hydroxide standard solution consumed in the blank test, measured in milliliters (mL);
c - potassium hydroxide standard solution, unit: moles per liter (mol/L);
m - Sample mass, unit: grams (g);
56.1 -- The molar mass of potassium hydroxide, measured in grams per mole (g/mol).
4.2 Atlas
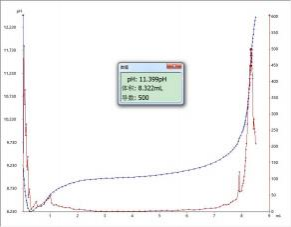
4.3 Conclusion
It can be seen from the measurement results that the determination of the acid value of capsicum red by potentiometric titration is simple to operate and the repeatability of the experimental results is good.
References
[1]GB 5009.229-2016 National Food Safety Standard - Determination of Acid Value in Foods.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094


