August 26, 2025
Tag:
I. Preface
Ethyleneimine (PEI) is a highly distinctive water-soluble polymer product. The primary amine, secondary amine, tertiary amine and other groups contained within its molecule endow PEI with very unique properties and extremely high chemical reactivity. Therefore, the related derivatives have jointly formed an independent, complete and distinctive water-soluble polymer product. It is widely applied in fields such as biomedicine, daily chemicals, coatings, inks, adhesives, electroplating, fiber treatment, sewage treatment, air purification, textile printing and dyeing, oilfield chemicals, paper chemicals, and metal surface processing. In this experiment, the JH-T6 fully automatic potentiometric titrator of Shanghai Jiahang was used to determine the endpoint according to its potential jump point and measure the content of tertiary amines to verify the feasibility of the experimental scheme.
Ii. Instruments and Reagents
2.1 Instruments
JH-T6 fully automatic potentiometric titrator, non-aqueous PH composite electrode, analytical balance, etc
2.2. Reagents
Anhydrous methanol, salicylaldehyde, 0.5mol/L hydrochloric acid standard titrant
Iii. Experimental Methods
3.1 Experimental Process:
Weigh about 0.15g of the original solution of polyethyleneimine, add 50mL of anhydrous methanol to dissolve the sample, then accurately transfer 5mL of salicylaldehyde with a 5mL pipette and add it to the titration cup. Place it on the titration stand, start stirring, and let it react for 10 minutes. Then, start the edited method and titrate with the calibrated 0.5mol/L hydrochloric acid titrant until the potential spike endpoint. Repeat the test in three groups and take the average value, which is the content of secondary and tertiary amines.
3.2 Instrument Parameters
The parameters of the instrument for testing the values of secondary and tertiary amines are shown in Table 1
Table 1 Parameter Settings of Titrator
|
Titration type: |
Dynamic titration |
Method Name: |
Determination of secondary and tertiary amine values of polyethyleneimine |
|
Burette volume: |
10mL |
Sample measurement unit: |
g |
|
Working electrode: |
PH Composite electrode |
Reference electrode: |
無 |
|
Stirring speed: |
7 |
Pre-stirring time: |
5s |
|
Electrode equilibrium time: |
8s |
Electrode equilibrium potential: |
1mv |
|
Titration speed:: |
slow |
Equilibrium potential before titration: |
6mv |
|
Display unit: |
PH |
Final volume: |
20mL |
|
Pre-titration addition volume: |
0(It can also be set appropriately) |
Minimum addition volume: |
0.02mL |
|
Potential spike: |
100mV |
Pre-controlled PH value: |
7 |
|
Correlation coefficient: |
56.1 |
Result unit: |
mg/g |
|
Titrant name: |
Hydrochloric acid |
Theoretical concentration: |
0.5444(Calibrated concentration) |
Iv. Results and Discussion
4.1 Experimental Results
The samples were tested and the experimental results are shown in Table 2
Table 2 Content Test Results
| Sample name |
Sampling volume/g |
c(HCL)/ mol/L |
Titration volumeV1/mL |
Secondary tertiary amine value/mg/g |
Average valuemg/g |
|
Polyethylene
Imine |
0.1643 |
0.5444 |
1.494 |
278.050 |
277.915 |
|
0.2079 |
1.893 |
278.085 |
|||
|
0.2217 |
2.016 |
277.719 |
4.2 Titration chromatogram
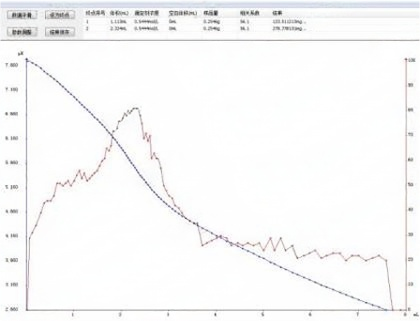
4.3. Conclusion
This test determined the content of tertiary amines in polyethyleneimine through potentiometric titration. The instrument's judgment reduced human errors and significantly improved the accuracy of the experiment. Therefore, potentiometric titration is a good choice for detecting such samples.
V. Precautions
When determining the values of secondary and tertiary amines, the reaction time for adding salicylaldehyde should not be too long. Within 10 to 20 minutes, the color should change from orange-yellow to yellow after addition, and a uniform yellow color should be observed. The reaction time for each time in this experiment is 10 minutes.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


